Home Research Publications Biography Contact
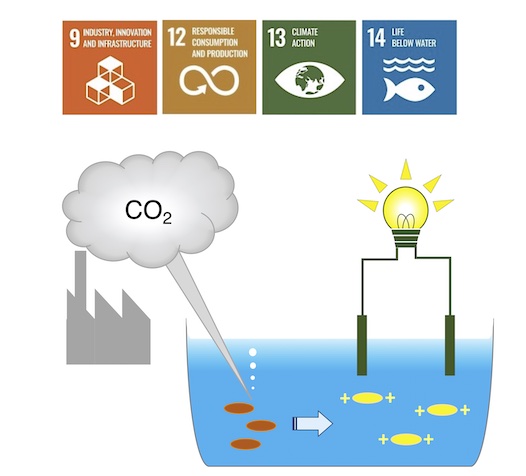
With the aim of reducing global warming due to intensified CO2 emissions, the Yoshida Lab created CO2 capture-induced electrolytes using tertiary diamines, such as N,N,Nf,Nf-tetramethyl-1,6-hexanediamine, N,N,Nf,Nf-tetramethyl-1,3-propanediamine, and bis(2-dimethylaminoethyl) ether. These diamines, soluble in water, effectively capture carbonic acid generated by introducing gaseous CO2, producing diammonium bicarbonate electrolytes. They exhibit electroconductivity dependent on the diaminefs basicity. Introducing argon into an electrolyte solution transforms the diammonium bicarbonate into its carbonate, and vice versa when reintroducing CO2, promising the repeatable use of the electrolytes.
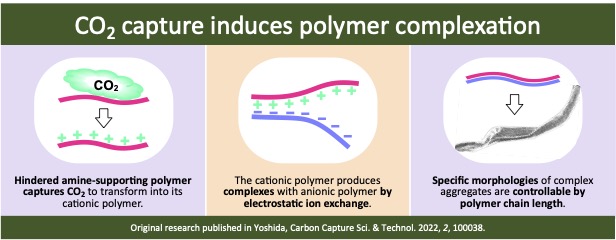
Another CO2 capture technology discovered by the Yoshida Lab involves carbonic acid capture-induced polymer complexes. A polymer supporting 2,2,6,6-tetramethylpiperidine, a hindered amine, captures carbonic acid in water upon introducing CO2. This polymer, containing the tetramethylpiperidinium bicarbonate, undergoes ion exchange with poly(sodium 4-styrenesulfonate) (PSS) to form insoluble complexes. These complexes vary in morphology depending on the PSS chain length: short PSS chains produce linear, rigid aggregates with a ribbon-like morphology, while long PSS chains yield planar aggregates. Capturing and storing carbonic acid in these polymer complexes contributes not only to preserving marine ecosystems from ocean acidification but also to creating new materials composed of carbonic acid.
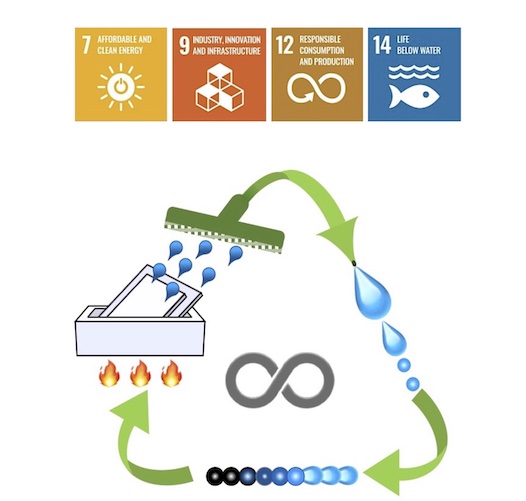
To address the intensifying issue of plastic waste, establishing closed-loop recycling systems is crucial. These systems involve recovering component monomers from waste plastics and utilizing them to reproduce the original products. The Yoshida Lab has established a convenient chemical recycling method for waste polystyrene foam using vacuum pyrolysis depolymerization and a spirit lamp flame. This method features simultaneous depolymerization and fractionation: the gaseous pyrolysates escape from the system, while the aromatic oligomers remain in the reactor, leading to high-purity monomer recovery. Applicable to all types of non-biodegradable waste plastics with carbon-carbon backbones, this method shows promise in reducing pollution caused by waste plastics. By returning waste plastics to their original fossil fuel state without the need for further fractionation and purification, it demonstrates the potential for waste plastics to serve as an alternative source for petroleum production, which could reduce natural fossil fuel consumption.
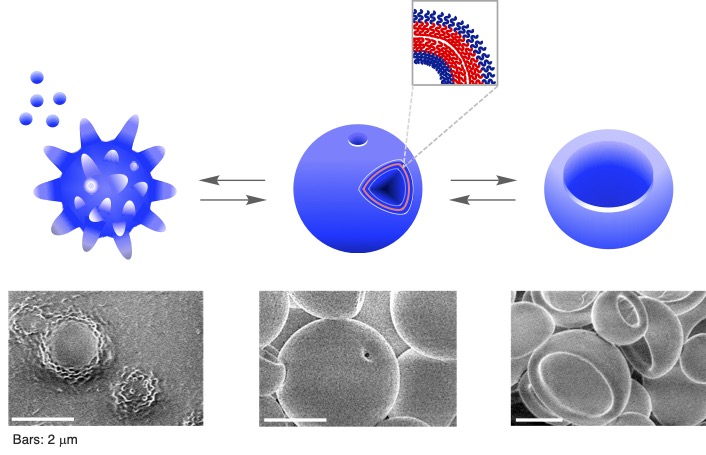
Micron-sized giant vesicles are viable artificial models for biomembranes of cells and organelles, based on their similarities in size and structure. The Yoshida Lab has fabricated artificial biomembrane models using synthetic polymer vesicles. These were prepared by the polymerization-induced self-assembly method, utilizing the nitroxide-mediated photo-controlled/living radical polymerization technique developed by this lab. The vesicles are composed of amphiphilic diblock copolymers, featuring a hydrophilic poly(methacrylic acid) block and a hydrophobic poly(alkyl methacrylate-random-methacrylic acid) block. The polymer vesicles can replicate static and dynamic morphologies of biomembranes, including villus-like structures for the digestive system, anastomosed tubular networks with fenestrated sheets for the endoplasmic reticulum and Golgi apparatus, perforated vesicles for the nuclear envelope, cup-shaped vesicles for the isolation membrane formed during the early stages of autophagy, neuron-like tubular extensions, and human erythrocyte-like morphology transformations. These findings contribute to understanding the differences between inanimate and animate matter, promising insights into the origin of life and the fabrication of artificial life using non-natural synthetic inanimate materials.
